|
ETHYLENE GLYCOL METHYL ETHER ACETATE |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
110-49-6 |
|
| EINECS NO. | 203-772-9 | |
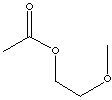
| FORMULA | CH3COOCH2CH2OCH3 | |
| MOL WT. | 118.13 | |
|
H.S. CODE |
2915.35 | |
| TOXICITY | ||
| SYNONYMS |
2-Methoxyethyl ester acetic acid; |
|
|
1-Acetoxy-2-methoxyethane; Methyl glycol monoacetate; 2-MEA; Methyl Cellosolve Acetate; 2-Methoxyethanol acetate; Ethylene glycol monomethyl ether acetate; Glycol Monomethyl Ether Acetate; 2-Methoxyethyl acetate; 2-Methoxyacetate Ethanol; Methyl Glycol Acetate; Ethylene glycol methyl acetate; Methoxyethylacetate; Methyl cellosolye acetaat; (Dutch); 2-Methoxy-ethyl acetaat (Dutch); Aethylenglykolmethylaetheracetat (German); Methyl glycol acetate (German); 2-Methoxyaethylacetat (German); Acetate de methyle glycol (French); 2-Methoxyethyle, acetate de (French); 2-Metossietilacetato (Italian); Acetate de 2-methoxyethyle; Acetate de l'ether monomethylique de l'ethylene-glycol (French); Acetato di metil cellosolve (Italian); beta-Methoxyethyl acetate; Acetic Acid, 2-methoxyethyl Ester; |
||
| DESCRIPTION | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
clear liquid, ether like odor |
|
| MELTING POINT |
-65 C |
|
| BOILING POINT |
145 C |
|
| SPECIFIC GRAVITY | 1.01 | |
| SOLUBILITY IN WATER | Miscible | |
| pH |
|
|
| VAPOR DENSITY |
|
|
| AUTOIGNITION |
|
|
| NFPA RATINGS | Health: 1; Flammability: 2; Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.402 |
|
| FLASH POINT |
44 C |
|
| STABILITY | Stable under ordinary conditions | |
| SALES SPECIFICATION | ||
| APPEARANCE |
clear liquid |
|
| PURITY |
99.7% min (ASTM E611) |
|
| WATER | 0.05% max ( ASTM D1364) | |
| SPECIFIC GRAVITY | 1.005 - 1.015 (ASTM D891) | |
| COLOR, APHA | 15 max | |
| DISTILATION RANGE | ||
| IBP | 145 C (ASTM D1078) | |
| DP | 149 C (ASTM D1078) | |
|
APPLICATIONS |
||
Glycol: any of a
class of organic chemicals characterized by having separate two hydroxyl (-OH)
groups, contribute to high water solubility, hygroscopicity and reactivity with
many organic compounds, on usually linear and aliphatic carbon chain. The
general formula is CnH2n(OH)2 or
(CH2)n(OH)2. The wider meaning
names include diols, dihydric alcohols, and dihydroxy alcohols. Polyethylene
glycols and polypropylene glycols are sometimes called polyglycols which are
derived by polymerization of ethylene oxide and propylene oxide respectively.
Polyethylene glycols are water-soluble at all molecular weights, but
polypropylene glycols become increasingly less water-soluble at high molecular
weights. Ethylene glycol, HOCH2CH2OH, is the simplest
member of the glycol family. Mono-, di- and triethylene glycols are the first
three members of a homologous series of dihydroxy alcohols. They are colourless,
essentially odourless stable liquids with low viscosities and high boiling
points. Ethylene glycol is a colourless, odourless, involatile and hygroscopic
liquid with a sweet taste. It is somewhat viscous liquid; miscible with water;
boiling point 198 C, melting point 13 C; soluble in ethanol, acetone, acetic
acid, glycerine, pyridine, aldehydes; slightly soluble in ether; insoluble in
oil, fat, hydrocarbones. It is prepared commercially by oxidation of ethylene at
high temperature in the presence of silver oxide catalyst, followed by hydration
of ethylene oxide to yield mono-, with di-, tri-, and tetraethylene glycols as
co-products. The yields of ethylene glycol are depend on pH conditions. The
acid-catalyzed condition in the presence of excess water provides the highest
yield of monoethylene glycol. Because of its low freezing point, involatility
and low corrosive activity, it is widely used in mixtures of automobile
antifreeze and engine-cooling liquids. Ethylene glycol has become increasingly
important in the plastics industry for the manufacture of polyester fibers and
resins, including polyethylene terephthalate, which is used to make plastic
bottles for soft drinks (PET bottles). MEG is the raw material in the production
of polyester fiber, PET resins, alkyd, and unsaturated polyester. Diethylene
glycol, CH2OHCH2OCH2CH2OH, is similar in
properties to MEG, but with a higher boiling point, viscosity, and specific
gravity. Diethylene glycol is used in the manufacture of unsaturated polyester
resins, polyurethanes and plasticizers. It is a water-soluble liquid; boiling
point 245 C; soluble in many organic solvents. It is used as a humectant in the
tobacco industry and in the treatment of corks, glue, paper and cellophane.
Diethylene glycol (DEG) is derived as a co-product with ethylene glycol and
triethylene glycol. The industry generally operates to maximize MEG production.
Ethylene glycol is by far the largest volume of the glycol products in a variety
of applications. Availability of DEG will depend on demand for derivatives of
the primary product, ethylene glycol, rather than on DEG market requirements.
Triethylene glycol, HO(C2H4O)3H, is a colourless,
odourless, non-volatile, and hygroscopic liquid. It is characterised by two
hydroxyl groups along with two ether linkages, which contribute to its high
water solubility, hygroscopicity, solvent properties and reactivity with many
organic compounds. DEG is used in the synthesis of morpholine and 1,4-dioxane.
TEG is displacing diethylene glycol in many of these applications on account of
its lower toxicity. TEG finds use as a vinyl plasticizer, as an intermediate in
the manufacture of polyester resins and polyols, and as a solvent in many
miscellaneous applications. Triethylene glycol (TEG) is derived as a coproduct
in the manufacture of ethylene glycol from ethylene oxide, and from "on-purpose"
TEG production using diethylene glycol. Some capacities are based on total
capacity for ethylene glycols. The main uses for TEG depend upon its hygroscopic
properties. Air conditioning systems use TEG as dehumidifiers and, when
volatilized, as an air disinfectant for bacteria and virus control. Glycols,
having high boiling point and affinity for water, are employed as liquid
desiccant for the dehydration of natural gas. The dehydration means the removal
of water vapor in refinery tower so that dry hydrocarbon gases can exit from the
top of the tower. There are wide range of glycol ethers which have bifunctional
nature of ether and alcohol. cellosolves are monoether derivatives of ethylene
glycol. They are excellent solvents, having solvent properties of both ethers
and alcohols. Glycol family products are versatile compounds used in the fields
include;
Glycol ethers, with the combination of ether, alcohol and hydrocarbon chain in one molecule, provide versatile solvency characteristics with both polar and non-polar properties. The chemical structure of long hydrocarbon chain resist to solubility in water, while ether or alcohol groups introduce the promoted hydrophilic solubility performance. This surfactant-like structure provides the compatibility between water and a number of organic solvents, and the ability to couple unlike phases. Glycol ethers are characterized by their wide range of hydrophilic/hydrophobic balances. glycol ethers are used as diluents and levelling agents in the manufacture of paints and baking finishes. Glycol ether series are used in the manufacture of nitrocellulose and combination lacquers. They are used as an additive in brake fluid. They are formulated for dying textiles and leathers and for insecticides and herbicides. They provides performance in cleaners products with oil-water dispersions. They are used in printing industries as they have a slow evaporation rate. They are used as a fixative for perfumes, germicides, bactericides, insect repellents and antiseptic. They are used as an additive for jet fuel to prevent ice buildup. Glymes, dimethyl ethers, have two terminal methyl groups which offer stability and high solvency. They are useful as solubilizers and phase transfer catalysts. Glymes offer the property required as an inert reaction medium chemical reaction due to their low chemical reactivity. They are suitable particularly for organometallic and polymerization reactions. Glycol ethers which contain hydroxyl group are also useful chemical intermediate. The hydroxyl group will undergo reaction with aldehydes (or ketones) to produce hemiacetals (or acetals), with epoxides to produce polyether alcohols, with halogenating agents to produce alkoxy alkyl halides, with carboxylic acid compounds or inorganic acids to produce a number of esters. Acetate is the ester that an organic group replaces a hydrogen atom in -OH group of acetic acid through reaction (typically condensation) with alcohols. Condensation is the reaction in which two molecules having -OH groups are joined with eliminating a water molecule from their -OH groups. They are produced by esterification reaction from acetic acid and the corresponding alcohol in the presence of strong acids like sulfuric acid. This reaction is reversible and acetate can be hydrolyzed back into alcohol and acetic acid in the presence of strong bases or strong acid, especially at elevated temperature. The term acetate is also for the salt that one or more of the hydrogen atoms of acetic acid are replaced by one or more cations of the base, resulting in a compound containing the negative organic ion of CH3COO-. Lower acetate is a non-polar to weak polar aprotic solvent which have some solubility portion in water. Its miscibility with water gets higher at elevated temperature. Higher acetates have a low solubility in water and used as extraction solvents for fine chemicals particularly for certain antibiotics. Organic acetates are good solvents for a broad range of resins as they are miscible with almost all common organic liquids. Due to their powerful solvency, high volatility and mild odor, acetates are widely used as solvents for paints, coatings, adhesives, cellulose, plastics, fats, wood stains. Additionally ether acetates series are also widely used as solvents. This surfactant-like structure provides the compatibility between water and a number of organic solvents, and the ability to couple unlike phases. The main products include ethyleneglycol monoethyl ether acetate, ethyleneglycol monobutyl ether acetate, and propyleneglycol monomethyl ether acetate. |
||
| TRANSPORTATION | ||
| PACKING | 200kgs in drum | |
| HAZARD CLASS | 3 | |
| UN NO. |
1189 |
|
| OTHER INFORMATION | ||
| Hazard Symbols: T, Risk Phrases: 10-20/21/22-60-61, Safety Phrases: 53-9-16-33-45 | ||